DNTP006494
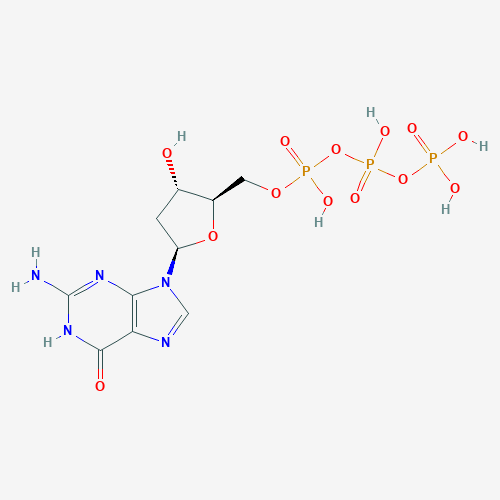
dGTP
| dNTP Pool | |
|---|---|
| Pool identifier | POOL001628 |
| Members of this Pool |
|
| Members of control pools |
|---|
| POOL001664 |
|
Last modified: 10/Sep/2021
| Results | |
|---|---|
| Publication |
Petrelli R, Meli M, Vita P, Torquati I, Ferro A, Vodnala M, D'Alessandro N, Tolomeo M, Del Bello F, Kusumanchi P, Franchetti P, Grifantini M, Jayaram HN, Hofer A, Cappellacci L: From the covalent linkage of drugs to novel inhibitors of ribonucleotide reductase: synthesis and biological evaluation of valproic esters of 3'-C-methyladenosine.,
Bioorg Med Chem Lett, 2014
PubMed |
| Value | 48 | Dimension | % |
| Error (standard deviation) | 5 |
| Relative compared to | control |
| Data presentation in publication | diagram |
| Source | |
|---|---|
| Source organism |
human
(Homo sapiens)
Taxonomy 9606 |
| Sample source | cell line |
| CLO Cell line name | HL-60 cell (CLO:0003775) |
| Cell line/type as in publication | HL-60 acute promyelocytic cell line |
| Compartment | whole cell |
| Experiment Details | |
|---|---|
| Measurement type | HPLC-UV |
| Extraction method | acid extraction |
| Remarks to measurement type | Hofer 1998. |
| Growth conditions | 0.3 mM A167 treatment |
| Treatment | |
|---|---|
| Drug/stress type | drug |
| Applied drug | 5'-O-valproyl-3'-C-methyladenosine (A167) |
| Treatment details/effects | Ribonucleotide reductase (RNR) inhibitor |
| Genes and Proteins | |
|---|---|
| No genes or proteins manipulated. | |